Active Cathodic Protection
https://en.wikipedia.org/wiki/Cathodic_protection
Definitions
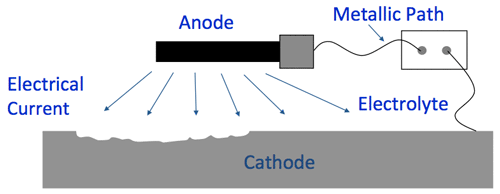
| Electrode | A substance that is a good conductor of electricity. An electrode acts as a metallic contact through which the current enters and leaves an electrolyte. |
| Anode | An electrode where oxidation occurs. In this context, it is a metallic structure that discharges DC current. Usually made of magnesium or zinc. Also known as sacrificial anode. |
| Cathode | An electrode where reduction occurs. In this context, it is the structure that needs to be protected from corrosion. |
| Electrolyte | A solution that contains salts and minerals. This medium is electrically conductive (sea water, for example). These are made up of atoms and ions can either gain or lose electrons. Electrolytes are also called ionic solutions. |
| Oxidation |
A chemical reaction where an electrode loses electrons during the reaction. This results in loss of electrons, gain of oxygen or loss of hydrogen. |
| Reduction |
A chemical reaction where an electrode gains electrons. This results in gain of electrons, loss of oxygen or gain or hydrogen. |
|
Redox (also known as oxidation-reduction) |
A chemical reaction that involves a transfer of electrons between two metals. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. |
|
Corrosion |
Corrosion of metal is a redox reaction in which a metal is oxidized naturally to its ions, resulting in partial or complete destruction of the metal. |
|
Rust |
Rust is an iron oxide formed by the reaction of iron and oxygen in the presence of water and/or air moisture. |
|
Cathodic Protection (CP)
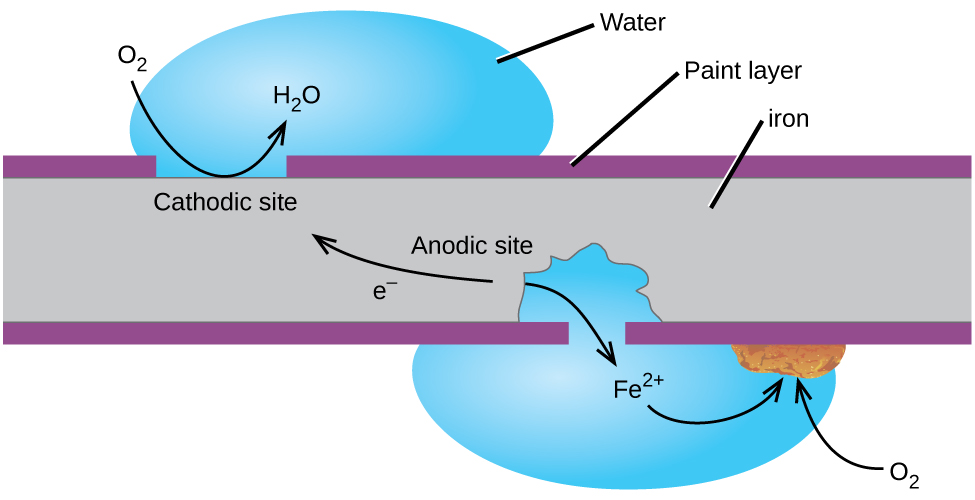
Cathodic Protection is a an electro-chemical process where DC current is applied to a metallic structure (made of iron or iron alloys) to stop it from corroding.
ForConsider an iron or steel rod submerged in water. Without cathodic protection, the rod begins to corrode or rust over time. When submerged in an electrolyte, sea water, for example, whenthe ironrod acts as an electrode that is inboth contacta withcathode water, it rusts. Rusting isand an electrochemicalanode. processParts thatof the rod act as cathode (called cathodic site) and some other parts, as an anode (anodic site). Once the protective paint on the surface of the rod wears away, corrosion begins whenand ironthis (Fe) isresults in contactformation withof water,rust.
To itsimplement electronscathodic toprotection, Oxygenintroduce (O2)an anode in the watervicinity of the structure that needs to formbe Ferrousprotected. Oxide.This Ferrousanode oxideneeds isto discharge electrical current (about 800 millivolts) to the firststructure. stepThe exact voltage needs to corrosion.
calculated on the salinity of the water around the rod and the distance between the anode and cathode.
Cathodic Protection cannot restore iron or steel that is already corroded. It can slow down or stop further corrosion of the structure.
The following is an illustration of how cathodic protection can be visualized for sea pods.